

Mohali: After innovative products like safety devices, sanitizers and masks to fight COVID epidemic, the National Institutes of Pharmaceutical Education and Research (NIPERs) have now come up with an immunity booster Herbal Tea to strengthen physical resistance against the disease which has claimed more than 4.7 lakh people across the globe. As no new effective […]

Geneva: After admitting that the world may have a Covid-19 vaccine within one year or even a few months earlier, the World Health Organization (WHO) on Friday said that UK-based AstraZeneca is leading the vaccine race while US-based pharmaceutical major Moderan is not far behind. WHO Chief Scientist Soumya Swaminathan stated that the AstraZeneca’s coronavirus […]
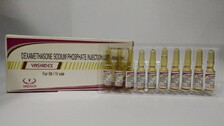
New Delhi: Dexamethasone, an inexpensive and widely used steroid, was included in the treatment protocol for COVID-19 patients in moderate to severe stages of illness by the Union health ministry on Saturday. The updated ‘Clinical Management Protocols for COVID-19’ advises the use of dexamethasone, which is already used in a wide range of conditions for […]

Hyderabad: Bharat Biotech, has successfully developed COVAXIN, India’s 1st vaccine candidate for COVID-19, in collaboration with the Indian Council of Medical Research (ICMR) – National Institute of Virology (NIV). The SARS-CoV-2 strain was isolated in NIV, Pune and transferred to Bharat Biotech. The indigenous, inactivated vaccine developed and manufactured in Bharat Biotech’s BSL-3 (Bio-Safety Level […]
New Delhi: Volunteers have begun participating in Brazil’s first clinical trial of Oxford COVID-19 vaccine. The ChAdOx1 vaccine technology is based on an adenovirus, and it is considered very safe, even in people with a weak immune system. The vaccine being used in the Brazilian trial, on 5,000 volunteers, is similar to the one used […]

New Delhi: After Bharat Biotech’s Covaxin, another potential COVID-19 vaccine indigenously developed by Ahmedabad-based Zydus Cadila Healthcare Ltd got nod from the Drugs Controller General of India (DCGI) on Thursday for human clinical trials, government sources said. The approval process was fast-tracked following a recommendation by the subject expert committee on COVID-19, considering the emergency […]

New Delhi: The Indian Council of Medical Research (ICMR) said that it has partnered with Bharat Biotech to fast-track clinical trials of India’s first indigenous COVID-19 vaccine Covaxin. ICMR Director General Balram Bhargava asserted that ICMR aims to launch the indigenous COVID-19 vaccine by August 15 after its completion of the clinical trials. In a […]
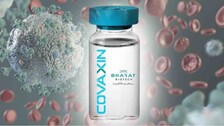
Courtesy: Gloria Methri, Republic The Indian Council of Medical Research (ICMR) has shortlisted 12 institutes, including one from Odisha, for human clinical trial of India’s first COVID-19 vaccine. ICMR has developed the indigenous BBV152 COVID vaccine in partnership with Bharat Biotech International Limited (BBIL). The apex body selected 12 institutes to fast track clinical trials […]
New Delhi: As soon as the Parliamentary standing committees started to resume work after a long spell of hibernation, it was not all good news that came in front of them. Notwithstanding the ambitious target set for its market launch, the Standing Committee on Science and Technology was told on Friday that India will have […]
Moscow: Russia has become the first nation to complete clinical trials of Covid-19 vaccine on humans, and the results have proven the medication’s effectiveness, the media reported on Sunday. Chief researcher Elena Smolyarchuk, who heads the Center for Clinical Research on Medications at Sechenov University, told Russian news agency TASS on Sunday that the human […]

New Delhi: Human clinical trials for a vaccine for COVID-19 has been initiated in the country with approximately 1,000 volunteers participating in the exercise for each of the two indigenously developed vaccine candidates, the ICMR said on Tuesday. Since India is one of the largest vaccine producers in the world, it is the country’s “moral […]
London: A Covid-19 vaccine developed by researchers at Oxford University generated an immune response against the disease in Phase-1 trial, The Telegraph reported even as official publication of the results are awaited. The Oxford vaccine candidate is believed to be leading the race among over 100 to find an effective protection against the disease. Oxford […]
New Delhi: At least seven Indian pharma companies are working to develop a vaccine against coronavirus as they join global efforts to find a preventive to check the spread of the deadly virus that has already infected more than 14 million globally. Bharat Biotech, Serum Institute, Zydus Cadila, Panacea Biotec, Indian Immunologicals, Mynvax and Biological […]
New York: Pfizer, which has struck a nearly $2 billion deal with the US government to deliver 100 million doses of a COVID-19 vaccine developed in collaboration with its German partner BioNTech, is hoping to seek regulatory approval for a vaccine “as early as October” this year and have a vaccine on the market by […]

Bhubaneswar: With COVID-19 cases rising exponentially in Bhubaneswar, the much awaited human trial of the vaccine against Coronavirus began at the Institute of Medical Sciences and SUM Hospital here on Monday. Covaxin, the vaccine being developed by Bharat Biotech, was administered to several persons who had volunteered to be part of the trial, informed Dr. E.Venkat […]

Copyright © 2024 - Odisha Television Limited All Rights Reserved.