

The US Food and Drug Administration (FDA) on Friday authorised emergency use of the Moderna Covid-19 Vaccine and the Pfizer-BioNTech vaccine for the prevention of Covid-19 in children down to 6 months of age.
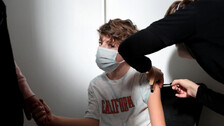
The decision came as the Omicron variant is spreading rapidly across the US and has led to record high hospitalisations among younger people.

Pfizer has said that Paxlovid reduces the risk of hospitalisation or death in Covid-19 patients by 89 per cent.

The pill has also been approved for those at high risk for progression to severe Covid-19, including hospitalisation or death.

In that study, the vaccine was 90.7 per cent effective in preventing Covid, according to the FDA.

"Covid-19 is "the eighth-highest killer of kids in this age group over the past year,"

New Delhi: The central drug regulator has given its go-ahead to a proposal by the Indian Council of Medical Research for the clinical trial of convalescent plasma in COVID-19 patients, as per the protocol developed by ICMR. The Drug Controller General of India said ICMR has submitted a list of institutes, which have shown an […]

New York: Blood plasma therapy that is being touted as the final resort to treat Covid-19 has not convinced the US Food and Drug Administration (FDA) as it has put the emergency use authorisation for blood plasma to treat the deadly respiratory disease on hold, the media reported. According to a report in The New […]

New York: The US Food and Drug Administration (FDA) has granted emergency use approval to the Regeneron Pharmaceuticals antibody treatment which was given to President Donald Trump after he was diagnosed with Covid-19. The emergency use authorisation is for the antibody cocktail casirivimab and imdevimab to be administered together — also known as REGN-COV2 or […]

New York: US-based drugmaker Moderna has confirmed that it has received a report from California’s health department that several individuals at one vaccination centre in San Diego were treated for possible allergic reactions after vaccination from one lot of the company’s Covid-19 vaccine. The company is “fully cooperating” with the California Department of Public Health […]

Copyright © 2024 - Odisha Television Limited All Rights Reserved.