

Ravi Vasanthapuram, the head of the research and development of TATA MD said the test kit is compatible with all standard Real-Time PCR Machines. It kit can detect the Omicron variant as well as other variants of SARS-CoV2 reported so far.
The DCGI gave its approval Friday, the source said.

The French pharma company Sanofi and its British peer GSK aim to produce up to one billion doses in 2021.

Hyderabad-based Biophore India Pharmaceuticals announced on Friday that it has applied for DCGI emergency use approval of Aviptadil Inhalation for marketing in India for the treatment of moderate to severe cases of Covid-19.
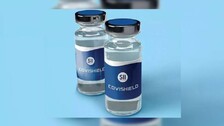
New Delhi: India’s drug regulator DCGI has extended the shelf life of Covishield, the Oxford-AstraZeneca COVID-19 vaccine, from six to nine months from its manufacturing date. In a letter to the Serum Institute of India, Drugs Controller General of India V G Somani said the SII is permitted to apply the shelf life of nine […]
New Delhi: India’s first indigenous mRNA vaccine candidate has received approval from the Drug Controller General of India to initiate Phase I/II human clinical trial, the Ministry of Earth Sciences said on Friday. The novel mRNA vaccine candidate, HGCO19 has been developed by Gennova, a company based in Pune and supported with seed grant under […]

Copyright © 2024 - Odisha Television Limited All Rights Reserved.