

Bhubaneswar: As many as 2 lakh more doses of Covishield vaccine will reach Odisha today. The State currently has a stock of 3.1 lakh shots of the vaccine which will…
New Delhi: The Post Graduate Institute of Medical Education and Research (PGIMER) in Chandigarh will be beginning the Phase II trial of oxford Covid-19 vaccine candidate — Covishield from the first week of September. The premier institute has received approval from its internal ethical committee and is awaiting the signing of the tripartite agreement between […]

Bhubaneswar: Odisha will receive nearly 5.4 lakh doses of Covid-19 vaccines in two phases during the ‘Tika Utsav’ that get kick-started today across the country. The State Health Department has…

November: Serum Institute of India (SII), the world’s largest vaccine manufacturer by volume, and Indian Council of Medical Research (ICMR), the apex body in India for biomedical research, announce completion of enrolment of phase 3 clinical trials for COVISHIELD in India. ICMR and SII have further collaborated for clinical development COVOVAX (Novavax) developed by Novavax, […]

Bhubaneswar: Around 700 vaccination centres were closed down in Odisha after the State ran out of Covid-19 vaccine stock on Wednesday. The vaccination drive was completely disrupted in Koraput district…
New Delhi: The Serum Institute of India (SII) has submitted additional data required by the Drug Controller General of India (DCGI) for determining the safety and immunogenicity of its Covid-19 vaccine candidate, Covishield, sources privy to the development told IANS. The data was submitted few days ago to the Central Drugs Standard Control Organisation (CDSCO), […]

New Delhi: Pune-based Serum Institute of India on Monday expressed hope at the possibility of Covid-19 vaccine – Covishield receiving the regulatory approval in “few days”, while emphasising that the company has a massive stockpile 40-50 million doses. The drug maker has partnered with global pharma giant AstraZeneca and the Oxford University for making the […]
New Delhi: Serum Institute of India’s (SII) COVID-19 vaccine candidate – Covishield – has been recommended by the Subject Expert Committee (SEC) which met today to examine the emergency-use authorisation (EUA) application sought by the pharmaceutical firm. With the SEC giving a green signal to Oxford-AstraZeneca’s COVID vaccine candidate, India is one step away from […]

New Delhi: The Subject Expert Committee of Central Drug Standard Control Organization on Saturday recommended Bharat Biotech’s ‘Covaxin’ for emergency use in India. However, the final decision on its approval will be taken by the Drug Controller General of India (DCGI). Expert panel recommends granting permission for restricted emergency use authorization for Bharat Biotech’s indigenously […]
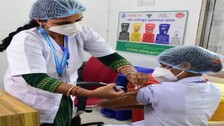
Bhubaneswar: As the country’s Covid-19 vaccination plan received a boost with the emergency use nod given to two vaccines – Covishield and Covaxin – by the Subject Expert Committee of the Central Drugs Standard Controller Organsiation (CDSCO), the day also saw a massive mock drill in as many as 125 districts spread across all the […]

New Delhi: Adar Poonawalla, CEO, Serum Institute of India (SII) said on Sunday that the Covishield, India’s first Covid-19 vaccine will roll out in the coming weeks. Poonawalla said SII risks on stockpiling the vaccine have paid off. His statement came minutes after the Drugs Controller General of India (DCGI) announced the approval for Covishield […]

New Delhi: Coronavirus vaccine developed by AstraZeneca and the University of Oxford will cost Rs 219-292 per shot to the government and will be priced at double that rate in private market ones such sales open up, said Adar Poonawalla, the CEO of its Indian manufacturer-Serum Institute of India, on Monday. Serum Institute of India […]

New Delhi: With the Drug Controller General of India (DCGI) approving Serum Institute of India’s ‘Covishield’ vaccine and Bharat Biotech’s ‘Covaxin’ for emergency use, paving way for their roll-out and administration to millions, Russian Sputnik V vaccine makers have said that they are working with AstraZeneca (AZ) to take the efficacy of AZ vaccine to […]

New Delhi: Vaccine makers Serum Institute of India and Bharat Biotech on Tuesday said both the companies jointly pledge for smooth roll out of COVID-19 vaccines in the country and the world. In a show of solidarity after some bitter comments, Adar Poonawala and Krishna Ella, jointly on behalf of Serum Institute and Bharat Biotech, […]
New Delhi: The Union health ministry has informed states and union territories that they are likely to receive the first supply of COVID-19 vaccine shortly and asked them to remain prepared to accept these consignments. In a communique, the ministry said vaccine will be supplied to the identified consignee points of 19 states and union […]

Copyright © 2024 - Odisha Television Limited All Rights Reserved.